
CDC Panel Backs Moderna ‘Spikevax’ for 18 and Older, as COVID Vaccine Injuries Continue to Rise, VAERS Data Show
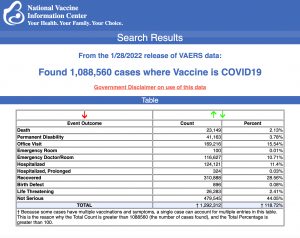
VAERS data released Friday by the Centers for Disease Control and Prevention included a total of 1,088,560 reports of adverse events from all age groups following COVID vaccines, including 23,149 deaths and 183,311 serious injuries between Dec. 14, 2020, and Jan. 28, 2022.
The Centers for Disease Control and Prevention (CDC) today released new data showing a total of 1,088,560 reports of adverse events following COVID vaccines were submitted between Dec. 14, 2020, and Jan. 28, 2022, to the Vaccine Adverse Event Reporting System (VAERS). VAERS is the primary government-funded system for reporting adverse vaccine reactions in the U.S.
The data included a total of 23,149 reports of deaths — an increase of 542 over the previous week — and 183,311 reports of serious injuries, including deaths, during the same time period — up 4,317 compared with the previous week.
Excluding “foreign reports” to VAERS, 747,760 adverse events, including 10,527 deaths and 69,089 serious injuries, were reported in the U.S. between Dec. 14, 2020, and Jan. 28, 2022.
Foreign reports are reports foreign subsidiaries send to U.S. vaccine manufacturers. Under U.S. Food and Drug Administration (FDA) regulations, if a manufacturer is notified of a foreign case report that describes an event that is both serious and does not appear on the product’s labeling, the manufacturer is required to submit the report to VAERS.
Of the 10,527 U.S. deaths reported as of Jan. 28, 19% occurred within 24 hours of vaccination, 23% occurred within 48 hours of vaccination and 60% occurred in people who experienced an onset of symptoms within 48 hours of being vaccinated.
In the U.S., 536.8 million COVID vaccine doses had been administered as of Jan. 28, including315 million doses of Pfizer, 204 million doses of Moderna and 18 million doses of Johnson & Johnson (J&J).
Every Friday, VAERS publishes vaccine injury reports received as of a specified date. Reports submitted to VAERS require further investigation before a causal relationship can be confirmed. Historically, VAERS has been shown to report only 1% of actual vaccine adverse events.
U.S. VAERS data from Dec. 14, 2020, to Jan. 28, 2022, for 5- to 11-year-olds show:
- 7,391 adverse events, including 159 rated as serious and 3 reported deaths.
The most recent death involves a 7-year-old girl (VAERS I.D. 1975356) from Minnesota who died 11 days after receiving her first dose of Pfizer’s COVID vaccine when she was found unresponsive by her mother. An autopsy is pending.
- 14 reports of myocarditis and pericarditis (heart inflammation).
- 26 reports of blood clotting disorders.
U.S. VAERS data from Dec. 14, 2020, to Jan. 28, 2022, for 12- to 17-year-olds show:
- 28,449 adverse events, including 1,609 rated as serious and 37 reported deaths.
The most recent deaths involve a 13-year-old male (VAERS I.D. 2042005) from an unidentified state who died from a sudden heart attack seven months after receiving his second dose of Moderna, and a 17-year-old female from an unidentified state (VAERS I.D. 2039111) who died after receiving her first dose of Moderna. Medical information was limited and it is unknown if an autopsy was performed in either case.
- 68 reports of anaphylaxis among 12- to 17-year-olds where the reaction was life-threatening, required treatment or resulted in death — with 96% of cases
attributed to Pfizer’s vaccine. - 615 reports of myocarditis and pericarditis with 603 cases attributed to Pfizer’s vaccine.
- 155 reports of blood clotting disorders, with all cases attributed to Pfizer.
U.S. VAERS data from Dec. 14, 2020, to Jan. 28, 2022, for all age groups combined, show:
- 19% of deaths were related to cardiac disorders.
- 54% of those who died were male, 41% were female and the remaining death reports did not include the gender of the deceased.
- The average age of death was 72.3.
- As of Jan. 28, 4,992 pregnant womenreported adverse events related to COVID vaccines, including 1,597reports of miscarriage or premature birth.
- Of the 3,509 cases of Bell’s Palsy reported, 51% were attributed to Pfizer vaccinations, 40% to Moderna and 8% to J&J.
- 850 reports of Guillain-Barré syndrome(GBS), with 41% of cases attributed to Pfizer, 30% to Moderna and 28% to J&J.
- 2,300 reports of anaphylaxis where the reaction was life-threatening, required treatment or resulted in death.
- 1,560 reports of myocardial infarction.
- 12,864 reports of blood clotting disorders in the U.S. Of those, 5,730 reports were attributed to Pfizer, 4,580 reports to Moderna and 2,506 reports to J&J.
- 3,878 cases of myocarditis and pericarditis with 2,387 cases attributed to Pfizer, 1,313 cases to Moderna and 166 cases to J&J’s COVID vaccine.
CDC advisory panel votes unanimously to recommend Moderna ‘Spikevax’ vaccine for 18 and older
The CDC’s Advisory Committee on Immunization Practices (ACIP) today voted 13-0 to recommend Moderna’s Spikevax COVID vaccine for adults 18 and older.
Dr. Rochelle Walensky, the agency’s director, is expected sign off on the recommendation, making it official.
The vote came after the FDA on Monday granted full approval of the Spikevax vaccine making it the second fully licensed COVID vaccine in the U.S.
However, similar to the licensing last year of Pfizer’s Comirnaty vaccine, the FDA approval and CDC recommendation of the Moderna vaccine raised a number of legal questions.
According to the FDA, Spikevax “has the same formulation as the [Emergency Use Authorization (EUA)] Moderna COVID-19 Vaccine and … can be used interchangeably with the EUA Moderna COVID-19 Vaccine to provide the COVID-19 vaccination series.”
However, in its approval letter, the FDA said Spikevax is “legally distinct” from the Moderna EUA vaccine.
The FDA made the same distinction between the Pfizer-BioNTech EUA vaccine and the Pfizer Comirnaty vaccine, which the agency fully licensed in August, 2021.
In the case of both Comirnaty and Spikevax, the FDA and the manufacturers of the vaccines acknowledge the fully licensed versions of their products are not available for distribution in the U.S. — a fact that caused some observers of today’s meeting to question the rush to approve it.
Dr. Meryl Nass, who listened to today’s meeting, said while the committee voted unanimously to recommend Spikevax fully, ”No one mentioned that it won’t be available and so nothing just happened.”
Nass said:
“Strangely, not a single ACIP member, nor their CDC briefers, acknowledged there was no plan to make the vaccine available to Americans in the near term. Therefore, the people making this decision have either been kept in the dark, or are trying to keep us in the dark. No one admitted that the purpose of today’s recommendation was to convince people they were getting a licensed product when they would instead get an EUA.”
Dr. Madhava Setty also questioned the panel’s decision, not only because the fully licensed Spikevax product isn’t available, but also because it was designed in response to earlier variants of the virus.
Setty said:
“Perhaps the biggest elephant in that room was that the data presented was outdated and did not reflect the vaccine’s effectiveness against Omicron, the dominant strain in this country at this time. What then is the purpose of approving this product? We learned later the reasons were clear: to extinguish vaccine hesitancy.”
Spikevax is a two-dose primary series, approved also for administration as part of a heterologous (“mix and match”) single booster dose for individuals who previously completed their original series of vaccinations with the Pfizer or J&J COVID vaccines.
Moderna awaits authorization for teens as FDA reviews heart inflammation risk
Moderna is still waiting for health agencies to authorize its vaccine for use in teenagers 12 to 17 years old.
The CDC met today to review the risk of what the agency characterized as a “rare” but serious form of heart inflammation affecting mostly young men after receiving an mRNA vaccine.
Moderna applied for Emergency Use Authorization in June for the adolescent age group, but the FDA told the company in October its review of the vaccine for kids wouldn’t be finished before January as it examined the risk of myocarditis in vaccines based on mRNA technology.
The FDA, in a statement to CNBC on Wednesday, said it is conducting the review as fast as possible, but it cannot predict how long the evaluation will take.
290 fully vaccinated residents in Massachusetts die of COVID over 1 week
Nearly 300 residents in Massachusetts died of COVID over the past week despite being fully vaccinated.
According to the International Business Times, between Jan. 22-29, Massachusetts health officials reported 290 additional breakthroughCOVID deaths, bringing the state’s total death toll among the fully vaccinated to 1,789. The figure represents 0.03% of the state’s vaccinated population.
During the same period, health officials recorded 27,530 new breakthrough infections and 555 additional hospitalizations. The state has now reported a total of 422,132 cases and 6,440 admissions among the fully vaccinated.
CDC admits natural immunity trumps vaccine immunity
A Jan. 19 report from the CDC showed natural immunity against COVID was at least three times as effective as vaccination alone at preventing people from becoming infected with the Delta variant.
Overall, the study showed natural immunity outperformed vaccine immunity when it came to preventing infection and hospitalization from Delta.
The results contradicted a previous CDC study, published in August 2021, which concluded vaccination was better than natural immunity.
The CDC issued a media statement about the August study, which was widely covered by mainstream press, but when a much larger Israeli study was published two weeks later with different findings, the agency did not acknowledged the new analysis.
Shaquille O’Neal says COVID vaccines should not be forced
Former Lakers star Shaquille O’Neal, who has been a vocal supporter of COVID vaccines for athletes, spoke out Thursday against vaccine mandates, Sports Illustrated reported.
Speaking with Entertainment Tonight’s Nischelle Turner on “The Big Podcast with Shaq,” O’Neal said he is not against the vaccine, but people should not have to choose between keeping their jobs and getting vaccinated.
“Look, I encourage everybody to be safe and take care of your family, I do,” O’Neal said, “but there’s still some people that don’t wanna take it. And you shouldn’t have to be forced to take something you don’t want.”
Turner stated her employer, CBS, has a vaccine mandate for workers. O’Neal responded, “That’s forced.”
When Turner disagreed, O’Neal said: “It is forced. Because if the man don’t take it, the man gonna get fired.”
O’Neal’s new position comes after he previously slammed Kyrie Irving for not getting vaccinated. In September, O’Neal said on his podcast if he was a teammate with Irving, he would have told him to “get his a!! up out of here.”
Source: the defender children health defense news and views by Megan Redshaw
